04 Nov 2020 by AXXELIS
By Henning Schmidt
To successfully turn a biologically active molecule into a beneficial and commercially successful medicine, many important decisions need to be taken along the way. These decisions are about choices that will drive development and commercialization strategy. The masses of data and parameters can make the required analysis very challenging. Particularly in resource-constrained environments and under time pressure.
During the preclinical stages of drug development, these decisions are mainly centred on compound selection and the first in human starting dose. For compounds reaching the clinical stages of development, decisions involve topics such as indication, patient, dose, administration route, and regimen selection, as well as, optimization of study design to allow for maximization of information content in the data under budget and time constraints. These decisions are up to the end of Phase 2b focused on minimizing the unknowns in order to confidently design the Phase 3 studies, allowing for an adequate expected probability of success, both in terms of meeting registration and subsequent marketing requirements.
To leverage the highly heterogeneous data within a drug development project (e.g. literature paper, in vitro data, in vivo data, clinical data, marketing information, competitor information, etc.) is challenging. In order to ensure informed decision making, all pieces of the puzzle need to be put together and knowledge gaps need to be identified as early as possible. Understanding of the big picture and quantification of the uncertainties is essential to be able to take action when risks become too high and to finally increase the level of confidence in the decisions. As a complicating factor, unmet medical needs and commercial interests urge to a time-efficient drug development process.

Pharmacometrics allows to systematically and quantitatively incorporate heterogeneous data and information, which is sometimes generated from different development programs, compounds, and biology. This is done by mathematical characterization of relationships of interest, such as dose, exposure, therapeutic and adverse effects. Pharmacometrics encompasses exposure-response models, quantitative drug-disease-trial models, clinical trial simulations, and enrichment or adaptive study designs. Uncertainties about known mechanisms are quantified as parameter uncertainties. For missing information on relationships between the steps from dose to effect or clinical benefit, models on alternative hypotheses can be formulated. Thanks to its mathematical characterization of these relationships, it allows the assessment of “what if” scenarios and to test different candidate assumptions.
In summary, pharmacometric analyses play an increasingly important role in support of answering key questions during drug development, allowing informed and more confident decision making.
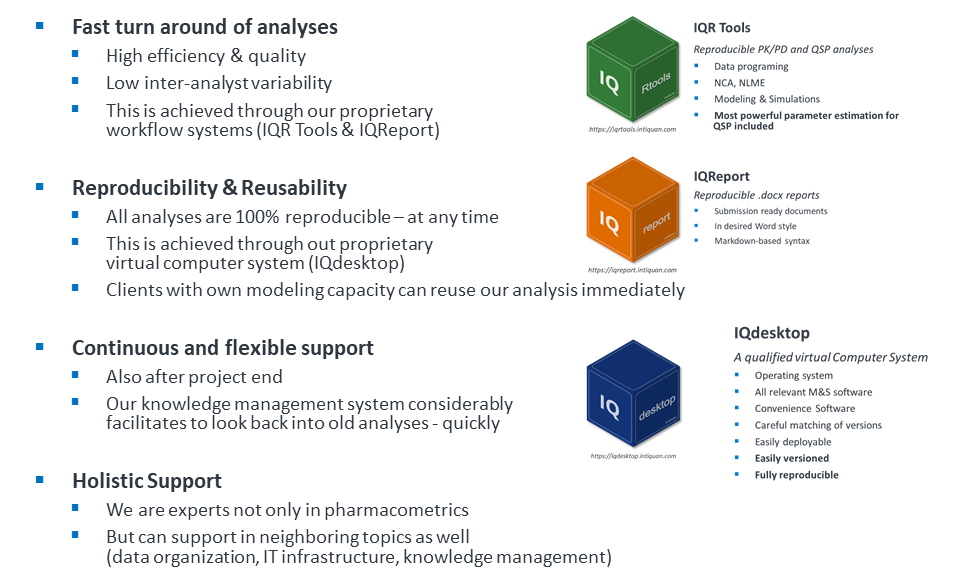
IntiQuan GmbH, was established in 2015 in Basel, Switzerland and is part of the AXXELIS expert network. Today IntiQuan plays an important role within the area of pharmacometric consulting services and holds a strong reputation based on software tools and workflow approaches supporting efficient modeling and simulation. As the time component in answering of important questions is key and pharmacometric analyses often can take an excessive amount of time, IntiQuan has invested a huge effort in streamlining analysis processes. Internally developed workflow tools considerably increase efficiency from initial data handling to the final reporting. The IntiQuan team has successfully supported over 100 projects with more than 15 clients in big pharma, biotech and non-profit organizations. Clients value especially the thoroughness of IntiQuan analyses, that in addition to meeting highest quality standards also are conducted in the most efficient manner in the pharmacometric world.
Case studies
Supporting Phase 2b design decisions
Pharmacokinetic data in healthy volunteers (Phase 1) and patients (Phase 2a) in response to intravenous/subcutaneous and intravenous doses, respectively, as well as efficacy readout in patients (Phase 2a) was available. The clinical team needed to decide on the most-likely effective dose and dosing regimen to inform the design for the upcoming Phase 2b study with more confidence.
This project supported the evaluation of potential pharmacokinetic differences between healthy volunteers and patients, the determination of subcutaneous drug absorption properties, establishment of a target concentration based on efficacy considerations, and determination of a dose and a dosing regimen that would achieve this target concentration.
The available data were integrated by a pharmacometric approach. The dose-concentration and concentration-efficacy response relationships were characterized. Key aspects like pharmacokinetic differences between healthy volunteers and patients, the determination of subcutaneous drug absorption properties needed to be accounted for to reliably translate doses to concentrations. Based on the quantification of the concentration-efficacy a target concentration leading to the desired efficacy was established. Candidate dose scenarios in patients were assessed via model-based simulations to determine the dose range to be tested in Phase 2b.
This analysis increased the understanding of the patient pharmacokinetics, supported dose and regimen selection, and provided crucial information that allowed to ensure maximal information to be expected from the planned Phase 2b study.
Support selection of First-in-Human dose
In preclinical studies potential non-linearities had been observed in the pharmacokinetics of the compound which complicated the first in human dose selection. Thus, the source of the potential non-linearities and whether it could be expected that they would translate to human needed to be evaluated and quantified to scale the preclinical doses to human.
Pharmacokinetic data in mice, rat, and dog from more than 10 studies under intravenous and oral administration were used. In addition, efficacy and toxicity study data were available in animals allowing to assume or derive potential concentration thresholds for efficacy and safety. The available data were all integrated in a cross-species pharmacokinetic model. It could clearly be shown that the potentially observed non-linearities in animal would not be expected in human. The animal to human translation was performed via a weight-based allometric scaling. Model-based simulations were used to assess candidate dose scenarios in humans.
The selected approach refined the assumption around the human pharmacokinetics based on animal data and lead to a more informed dose selection. The pharmacometric approach was also used to improve the FIH study design with respect to PK sampling times, follow-up time, etc.